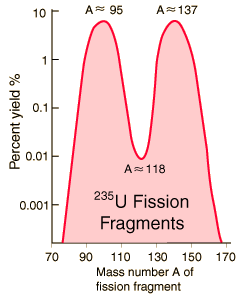
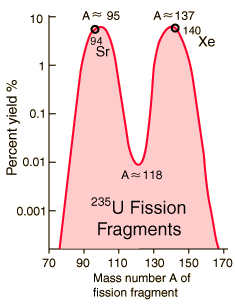
Fission Fragments
| Index Fission concepts | ||
| Go Back |
Fission Fragment Example
| Index Fission concepts | ||
| Go Back |
Fission Fragment DecayThis particular set of fragments from uranium-235 fission undergoes a series of beta decays to form stable end products.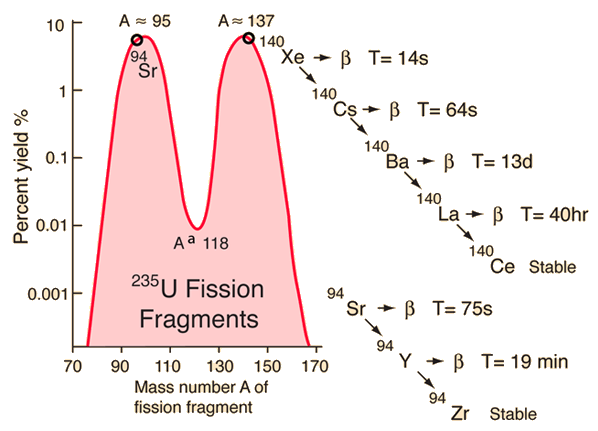 | Index | ||
| Go Back |
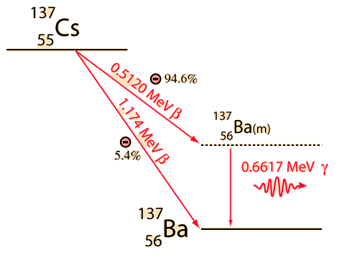
Cesium-137Cesium-137 and strontium-90 are the most dangerous radioisotopes to the environment in terms of their long-term effects. Their intermediate half-lives of about 30 years suggests that they are not only highly radioactive but that they have a long enough halflife to be around for hundreds of years. Iodine-131 may give a higher initial dose, but its short halflife of 8 days ensures that it will soon be gone. Besides its persistence and high activity, cesium-137 has the further insidious property of being mistaken for potassium by living organisms and taken up as part of the fluid electrolytes. This means that it is passed on up the food chain and reconcentrated from the environment by that process.
References: Environmental Protection Agency bulletin. Wiki: Cesium-137 | Index | ||
| Go Back |
Strontium-90Strontium-90 and cesium-137 are the radioisotopes which should be most closely gaurded against release into the environment. They both have intermediate halflives of around 30 years, which is the worst range for half-lives of radioactive contaminants. It ensures that they are not only highly radioactive but also have a long enough halflife to be around for hundreds of years. Strontium-90 mimics the properties of calcium and is taken up by living organisms and made a part of their electrolytes as well as deposited in bones. As a part of the bones, it is not subsequently excreted like cesium-137 would be. It has the potential for causing cancer or damaging the rapidly reproducing bone marrow cells.Strontium-90 is not quite as likely as cesium-137 to be released as a part of a nuclear reactor accident because it is much less volatile, but is probably the most dangerous component of the radioactive fallout from a nuclear weapon. Strontium-90 undergoes beta decay, emitting electrons with energy 0.546 MeV with a half-life of 28.8 years. The decay product is yttrium-90. References: Environmental Protection Agency bulletin. Wiki: Strontium-90
| Index | ||
| Go Back |
Iodine-131Iodine-131 is a major concern in any kind of radiation release from a nuclear accident because it is volatile and because it is highly radioactive, having an 8 day half-life. It is of further concern in the human body because iodine is quickly swept up by the thyroid, so that the total intake of iodine becomes concentrated there. The thyroid has a maximum uptake of iodine, however, so some protection against iodine releases can be afforded by taking potassium iodide tablets to load up the thyroid to capacity so that radioactive iodine would be more likely to be excreted.References: Environmental Protection Agency bulletin. Wiki: Iodine-131 | Index | ||
| Go Back |
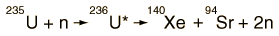
No comments:
Post a Comment