Electromagnetic Spectrum
The electromagnetic (EM) spectrum is the range of all types of EM radiation. Radiation is energy that travels and spreads out as it goes – the visible light that comes from a lamp in your house and the radio waves that come from a radio station are two types of electromagnetic radiation. The other types of EM radiation that make up the electromagnetic spectrum are microwaves, infrared light, ultraviolet light, X-rays and gamma-rays.You know more about the electromagnetic spectrum than you may think. The image below shows where you might encounter each portion of the EM spectrum in your day-to-day life.
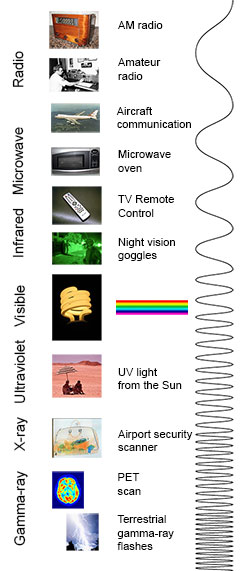
The electromagnetic spectrum from lowest energy/longest wavelength (at the top) to highest energy/shortest wavelength (at the bottom). (Click image for a larger version.)
Radio: Your radio captures radio waves emitted by radio stations, bringing your favorite tunes. Radio waves are also emitted by stars and gases in space. Microwave: Microwave radiation will cook your popcorn in just a few minutes, but is also used by astronomers to learn about the structure of nearby galaxies.
Infrared: Night vision goggles pick up the infrared light emitted by our skin and objects with heat. In space, infrared light helps us map the dust between stars.
Visible: Our eyes detect visible light. Fireflies, light bulbs, and stars all emit visible light.
Ultraviolet: Ultraviolet radiation is emitted by the Sun and are the reason skin tans and burns. "Hot" objects in space emit UV radiation as well.
X-ray: A dentist uses X-rays to image your teeth, and airport security uses them to see through your bag. Hot gases in the Universe also emit X-rays.
Gamma ray: Doctors use gamma-ray imaging to see inside your body. The biggest gamma-ray generator of all is the Universe.
Infrared: Night vision goggles pick up the infrared light emitted by our skin and objects with heat. In space, infrared light helps us map the dust between stars.
Visible: Our eyes detect visible light. Fireflies, light bulbs, and stars all emit visible light.
Ultraviolet: Ultraviolet radiation is emitted by the Sun and are the reason skin tans and burns. "Hot" objects in space emit UV radiation as well.
X-ray: A dentist uses X-rays to image your teeth, and airport security uses them to see through your bag. Hot gases in the Universe also emit X-rays.
Gamma ray: Doctors use gamma-ray imaging to see inside your body. The biggest gamma-ray generator of all is the Universe.
Is a radio wave the same as a gamma ray?
Are radio waves completely different physical objects than gamma-rays? They are produced in different processes and are detected in different ways, but are they are not fundamentally different. Radio waves, gamma-rays, visible light, and all the other parts of the electromagnetic spectrum are electromagnetic radiation.Electromagnetic radiation can be described in terms of a stream of mass-less particles, called photons, each traveling in a wave-like pattern at the speed of light. Each photon contains a certain amount of energy. The different types of radiation are defined by the the amount of energy found in the photons. Radio waves have photons with low energies, microwave photons have a little more energy than radio waves, infrared photons have still more, then visible, ultraviolet, X-rays, and, the most energetic of all, gamma-rays.
Measuring electromagnetic radiation
Electromagnetic radiation can be expressed in terms of energy, wavelength, or frequency. Frequency is measured in cycles per second, or Hertz. Wavelength is measured in meters. Energy is measured in electron volts. Each of these three quantities for describing EM radiation are related to each other in a precise mathematical way. But why have three ways of describing things, each with a different set of physical units?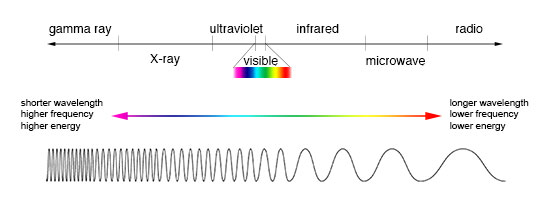
Comparison of wavelength, frequency and energy for the electromagnetic spectrum. (Click image for a larger version.)
Astronomers who study radio waves tend to use wavelengths or frequencies. Most of the radio part of the EM spectrum falls in the range from about 1 cm to 1 km, which is 30 gigahertz (GHz) to 100 kilohertz (kHz) in frequencies. The radio is a very broad part of the EM spectrum.
Infrared and optical astronomers generally use wavelength. Infrared astronomers use microns (millionths of a meter) for wavelengths, so their part of the EM spectrum falls in the range of 1 to 100 microns. Optical astronomers use both angstroms (0.00000001 cm, or 10-8 cm) and nanometers (0.0000001, or 10-7, cm). Using nanometers, violet, blue, green, yellow, orange, and red light have wavelengths between 400 and 700 nanometers. (This range is just a tiny part of the entire EM spectrum, so the light our eyes can see is just a little fraction of all the EM radiation around us.)
The wavelengths of ultraviolet, X-ray, and gamma-ray regions of the EM spectrum are very small. Instead of using wavelengths, astronomers that study these portions of the EM spectrum usually refer to these photons by their energies, measured in electron volts (eV). Ultraviolet radiation falls in the range from a few electron volts to about 100 eV. X-ray photons have energies in the range 100 eV to 100,000 eV (or 100 keV). Gamma-rays then are all the photons with energies greater than 100 keV.
Why do we put telescopes in orbit?
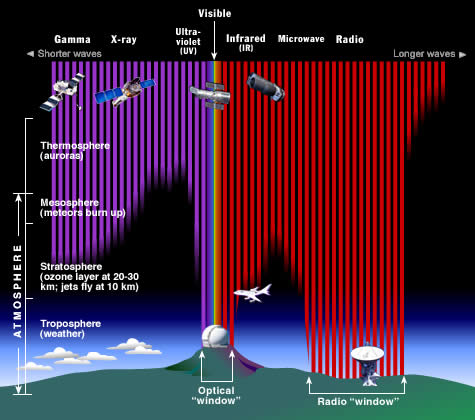
The Earth's atmosphere stops most types of electromagnetic radiation from space from reaching Earth's surface. This illustration shows how far into the atmosphere different parts of the EM spectrum can go into the atmosphere before being absorbed. Only portions of radio and visible light reach the surface. (Credit: STScI/JHU/NASA)
For long-term observations, however, it is best to have your detector on an orbiting satellite and get above it all!
Updated: March 2013
No comments:
Post a Comment